PROPERTIES OF POLYMERS
Heat capacity/ Heat conductivity –
conductivity The extent to which the plastic or polymer acts as an effective insulator against the flow of heat. (The polystyrene in disposable plastic glasses isn’t a very good insulator. However, blowing air through styrene while it is being polymerized gives the Styrofoam used for disposable coffee cups, which is a much better insulator.)
Thermal expansion –
expansion The extent to which the polymer expands or contracts when heated or cooled. (Silicone is often used to seal glass windows to their frames because it has a very low coefficient of thermal expansion.) Thermal expansion is also concerned with the question of whether the polymer expands or contracts by the same amount in all directions. (Polymers are usually anisotropic. They contain strong covalent bonds along the polymer chain and much weaker dispersive forces between the polymer chains. As a result, polymers can expand by differing amounts in different directions.)
Crystallinity –
Crystallinity The extent to which the polymer chains are arranged in a regular structure instead of a random fashion. (Some polymers, such as Silly Putty and Play Dough, are too amorphous and lack the rigidity needed to make a useful product. Polymers that are too crystalline often are also too brittle.)
Permeability –
Permeability The tendency of a polymer to pass extraneous materials. (Polyethylene is used to wrap foods because it is 4000 times less permeable to oxygen then polystyrene.)
Elastic modulus –
The force it takes to stretch the plastic in one direction.
Tensile strength –
The strength of the plastic. (The force that must be applied in one direction to stretch the plastic until it breaks.)
Resilience –
The ability of the plastic to resist abrasion and wear.
Refractive index –
The extent to which the plastic affects light as it passes through the polymer. (Does it pass light the way PMMA does, or does it absorb light like PVC?).
Resistance to electric current –
Is the material an insulator, like most polymers, or does it conduct an electric current? (There is a growing interest in conducting polymers, which can be charged and discharged, and photo conducting polymers that can pick up an electric charge when exposed to light.)
CLASSIFICATION OF POLYMER
Structure of Polymers :
Polymers are formed through the combination of monomers but monomers are not homogenous.
The composition of polymers vary and propagate different results, these outcomes are what we see today in our lives.
Based on Monomers :
As polymers are made up of monomers, monomers are not homogenous and hence produce different kinds of polymers.
Polymer made from one kind of monomer is called a homopolymer.
Polymer made from using 2 different monomers is called a copolymer.
Polymer made from using 3 different kinds of monomers is classified as a terpolymer.
Based on Origin :
Natural Polymers
As the name suggests, these polymers occur in nature like plants and animals. Common examples are proteins, cellulose, starch to name a few.
Synthetic Polymers
Polymers that are artificially made to serve human necessities. These polymers are artificially made in a lab and mass produced to serve varying industries. Example – Polyethylene is mass produced to serve packaging needs. Other examples would be PVC, synthetic rubber, Polystyrene.
Semi Synthetic Polymers
This kind of polymer are obtained from the synthesis of natural polymers to improve their physical properties through a chemical reaction in a controlled environment. A prime example would be Vulcanized Rubber used to make tyres.
Based on their structure polymer can be classified as
Linear Polymer –
The structure of linear polymer has a structure is that of a long straight chain without any side branches. The molecules are closely packed there by having a high level of density and strength. As the molecules are well bound it offers a high melting point. A common example is PVC; used to make pipes and cables, another example could be HDPE (High density Polyethylene).
Branched-Chain polymer
This type of polymer has a straight chain with branches that originate randomly on the linear chain.
The branches vary in length and as a result of these branches the molecules are not closely packed together. These polymers are of low density and low melting point. Cross linked polymer.
This type of structure involves 2 linear chain polymers linked together via covalent bonding. Picture a ladder where one side of the ladder (linear chain polymer) is linked with other side of the ladder by a step.
The step is the covalent bond while the sides of the ladder are the 2 linear chain polymers. These polymers are brittle and hard.
Another name for cross linked polymer is network polymers. A good example would be vulcanized rubber or Bakelite (used in electrical insulators).
Based on Polymerization :
Polymerization, as we know it, is the process by which monomers undergo a chemical reaction to bond and form a polymer chain. Based on how they are polymerized, the classification is as follows:
Addition Polymerization :
Polymers formed by the addition of monomers repeatedly without the removal of any by-product (water or alcohol). Basically, joining a lot of monomers together without losing its by products made as a result of the process. Example Polyethene is formed from the combination of ethene molecules.
Other examples can include PVC, Teflon and PP.
Condensation Polymerization :
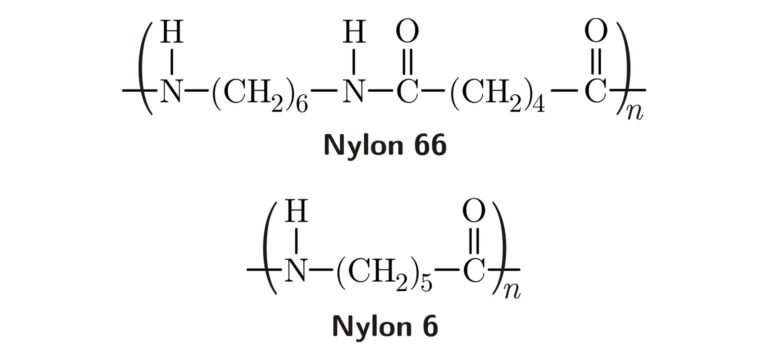
Polymers formed by the combination of monomers with the elimination of by-products or small molecules like water or alcohol. Ex. Nylon 66 is formed through condensation polymerization whereby water is eliminated from the process. Another example is PET.
Based on Molecular forces :
The properties of polymers are governed by intermolecular forces, e.g., Van der Waal’ s forces and hydrogen bonds, present in the polymer. These forces also bind the polymer chains.
Under this category, the polymers are classified into the following groups based on the magnitude of intermolecular forces present in them.
Elastomers
Elastomers are polymers with a unique property. They are rubber-like solid polymers.
Elastomers have shape memory meaning they can stretch or compress and still be able to return back to its original shape.
Elastomers consist of weak binding forces in their polymeric structure allowing them to reform their shape. Example – Natural rubber and Vulcanized rubber. Rubber bands are the most common example.
Thermoplastics
Polymers that can soften on heating and harden when cooled are termed as thermoplastic. The intermolecular forces weaken rapidly with increased temperature, yielding a viscous state allowing them to be reshaped into parts through plastic processing techniques like injection molding, extrusion etc.
Polyethylene, PVC, Polystyrene are some examples of thermoplastics.
Thermosetting polymers
Thermoset polymers are semi-liquid and possess low molecular masses, when exposed to high temperature they become hard and change irreversibly.
These polymers are cross linked molecules, which on heating undergo extensive cross linking and form a three-dimensional structure which is irreversible.
The best example to be used here is Bakelite.
Fibres
This class of polymers have a long thread like structure that have the ability to be woven into fibres or fabric. They possess strong intermolecular force of hydrogen bond giving them high tensile strength but low elasticity and low melting points.
Ex. Nylon 66 (Polyamide).
PLASTIC PROCESSING
Plastic processing is converting plastic raw materials into semi-finished goods through various techniques.
These techniques can be classified into
1. Primary Techniques: Injection, Extrusion, Blow, Compression and transfer moulding.
2. Secondary Processing Methods: Roto, Thermoforming, Coating, Casting, Fabrication and Calendaring etc.
3. Tertiary Processing Methods: Cutting, Drilling, Welding and Bending etc.
A plastic with good processability possesses the properties necessary to make it easy to process the plastics into desired shapes. The main characteristics or the properties which determine a plastic’s processability are molecular weight, uniformity, additive type, content and plastic feed rates.
Injection
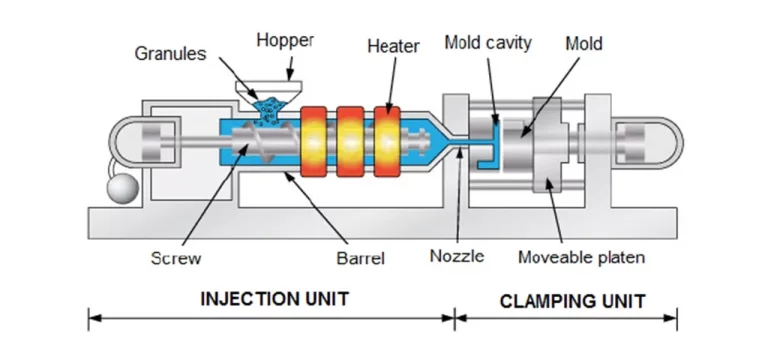
Injection molding is the widely used plastic processing technique for mass manufactured products, examples include chair, toys, cases for consumer electronics.
The basic idea is injecting molten plastic into a mould.
An injection machine is made up of three primary components – the feed hopper, the screw and the heated barrel. Plastic granules for the part are fed into the heated barrel by a hopper.
The material is then melted utilizing the frictional action of a reciprocating screw accompanied with heater bands. The molten plastic is then injected through the nozzle and into a mold cavity – it may seem easy, but injection molding is actually a very complex process. While in the mold cavity, the material cools and solidifies to the configuration of the cavity. When the part has hardened, the moveable platen that the mold is mounted opens and the part is ejected using ejector pins.
Injection molding machines are classified based on their clamping force in tonnage unit. Example – A machine that delivers 100 clamping force is rated as a 100 tons machine.
Too little or too much pressure can cause part quality issues.
Clamp force is required in order to keep the injection mold closed during the high-pressure injection molding process, which is the primary purpose of the clamp unit.
How does one estimate what injection machine should be used?
It’s all based on projection area of the product and multiplying that are by an injection mold clamp force between 2 to 8.
2 being the starting point for high melt flow material and 8 being the starting point for low melt flow.
For this example, polycarbonate has been selected as the material for molding. Polycarbonate is fairly stiff and a lower flow material, so the injection mold clamp factor used must be towards the high side. Injection mold clamp factor of 5 tons per square inch is adequate for polycarbonate. That means that the 36 square inch projected area found above must be multiplied by the injection mold clamp factor of 5 tons per square inch, to result in a total injection mold clamp tonnage requirement of 180 tons (36 x 5 = 180). There should be a safety factor of 10% added, so the final injection mold clamp force needed is 198 tons. The machine with the closest rating for this product would be a 200-ton machine.
These numbers are only correct if there is a shutoff land surrounding the part. If that land does not exist, the injection mold clamp tonnage will have to be doubled, tripled, or even more! This may result in mold damage, machine damage, and longer cycle times.
The purpose of this shutoff land is to concentrate all the clamp force to the area surrounding the cavity. That allows us to use less total force than if the clamp force is dispersed over the entire face of the mold base. Without the shutoff land, the amount of clamp force will be 3 to 4 times as much as with the shutoff land.
EXTRUSION MOLDING
Essentially, it is not much different from squeezing tooth paste out of the tube. Anything that is long with a consistent cross section is probably made by extrusion. Common examples are drinking straws, plumbing pipes, door insulation seals, optical fibers, etc.
Extrusion is a manufacturing process in which plastic granules are fed into a barrel conveyed through with the help of a screw and heat conductors around the screw. The heat melts the granules and is then forced through a die to form the shape of the expected product. Generally the shape is a long tube. The die is in the form of a cross section that determines the shape of the tube.
Once the granules are forced through a die, the tube is later cooled and hardened into a shape.
