About Polymers
What are Polymers?
Polymers are molecules that are chemically linked together. A polymer is basically a group of monomers, hence the name ‘Poly’mer.
The word Polymer comes from the Greek ”poly” meaning many, and ”meros” meaning parts or units. A polymer is a group of many units. You combine many monomers (one unit) to create a polymer
Imagine a paper clip as a monomer and a chain of paper clips linked together can be called polymer. To make this chain many monomers are chemically

A polymer can be a three dimensional network (think of the repeating units linked together left and right, front and back, up and down) or two-dimensional network (think of the repeating units linked together left, right, up, and down in a sheet) or a one-dimensional network (think of the repeating units linked left and right in a chain)
Plastic is made up of polymers and hence we can say that all plastic is polymers but not all polymers are plastic as even biological and inorganic molecules are
The common composition of polymers is of carbon and hydrogen. Polymers comprising of these elements are bonded together to form long chains that are called the backbone of polymers. Examples of polymers containing carbon and hydrogen are Polyethylene (PE), Polypropylene (PP) and Polystyrene (PS).
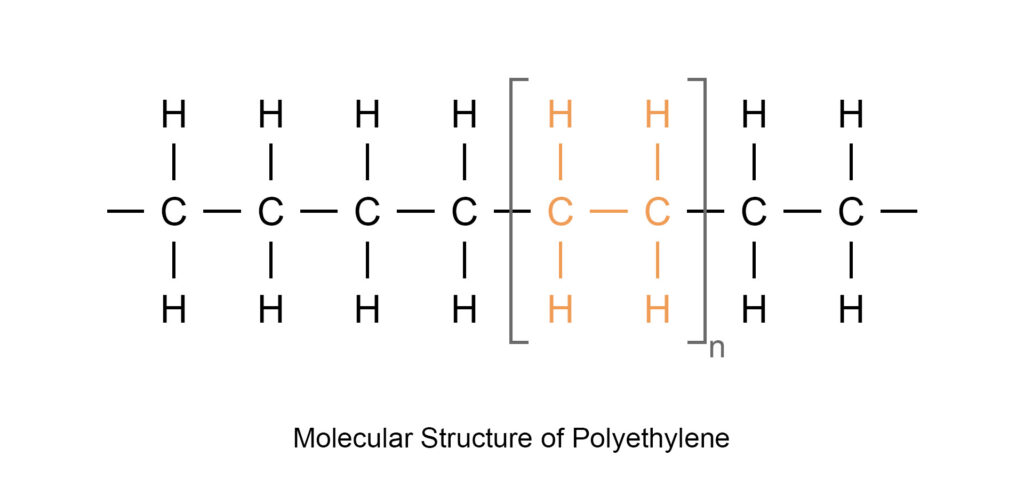
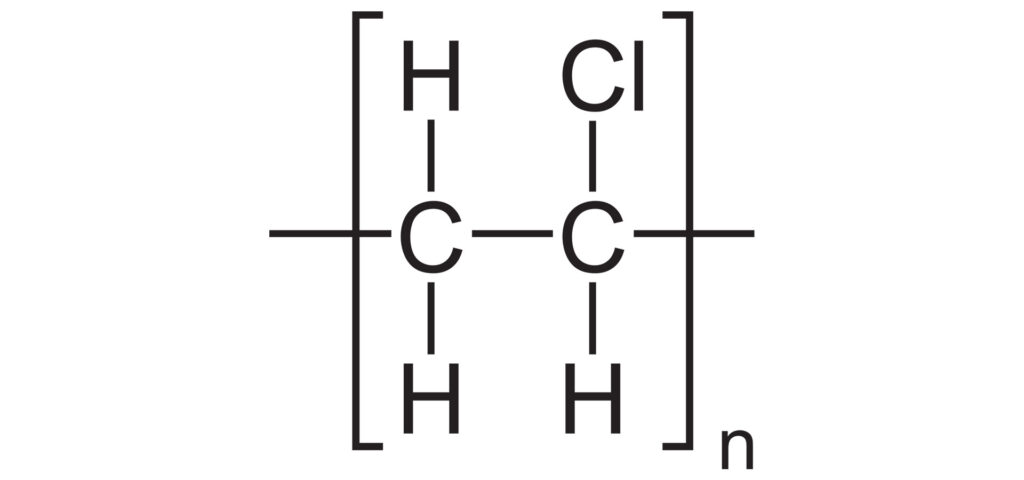
When Chlorine joins the all-carbon back-bone the polymer becomes Polyvinyl Chloride (PVC).
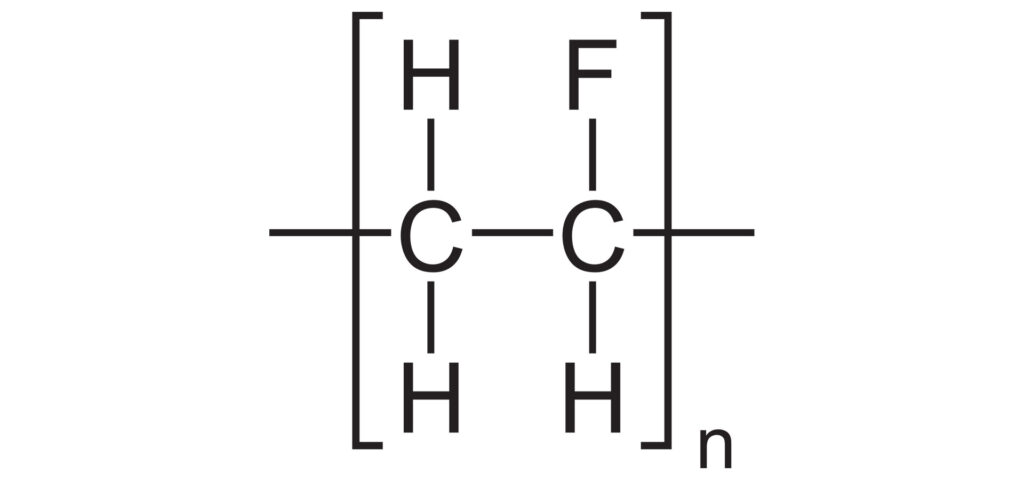
Replace Chlorine with Fluorine and we have Teflon.
Histroy of Polymers
The first plastic ever made was called Parkesine. It was synthesized from nitrocellulose and camphor. It could be crafted into a variety of shapes. It was created by Alexander Parkes in Birmingham, England, 1855.

Parkesine plastics were made by dissolving nitrocellulose (a flammable nitric ester of cotton or wood cellulose) in solvents such as alcohol or wood naphtha and mixing in plasticizers such as camphor.
In 1867 Parkes’s business partner, Daniel Spill, patented Xylonite, a more-stable improvement upon Parkesine. He later went on to found the Xylonite Company which produced molded objects such as chess pieces from his material.


Across the sea between 1868 and 1869, an American inventor, John Wesley Hyatt commercially improved Parkesine to initially develop a substitute for ivory billiard balls.
With this invention he went on to patent it under the name Celluloid and establish the Albany Billiard Ball Company to manufacture celluloid-material billiard balls. Along with his brother he also found The Hyatts’ Celluloid Manufacturing Company that produced a variety of products using the material. He was widely credited for bringing about a revolutionary change in the plastic world.

Legal disputes surged between Spill and Hyatt over the patent, but the judge ultimately ruled that neither of them had exclusive claims to it. The true inventor of celluloid/xylonite was Alexander Parkes, due to his mention of camphor in his earlier experiments and patents.
The first fully synthetic polymers were first made in 1907 with the introduction of Bakelite by Leo Baekeland. This contained no molecules derived from nature. It is a polymer with high resistance to heat and chemicals. It has low electrical conductivity due to which it is most commonly used for making electrical switches.
Bakelite was marketed as the ‘‘material of a thousand uses’’ because not only was it a good insulator it was also durable, heat resistant, and, unlike celluloid, ideally suited for mechanical mass production. Bakelite could be shaped or molded into almost anything, providing endless possibilities.
The success of Celluloid and Bakelite paved the way for major companies to develop new plastics that would go on to shape the world.
So how did the plastics we see today first emerge?
Let’s take a look..

NYLON
In the 1930s, Wallace Carothers of Dupont was investigating an alternative to silk. Wallace and his team were experimenting with hexamethylenediamine and mixing it with adipic acid. They then pulled strands from the concoction and spun them into plastic thread using a process called cold drawing. Six carbon monomers were formed called fiber 66. It was a strong, elastic and a very insoluble fiber with a high melting point.
It was first used commercially in a nylon-bristled tooth brush (1938), followed more famously by women’s stockings.


POLY VINYL CHLORIDE
PVC came into being in the year 1872 by a German scientist, Eugene Baumann by polymerization of vinyl chloride while it was exposed to sunlight. He didn’t patent it as he could identify commercial use for it
Not until Friedrich Klatte took out a patent on PVC but yet couldn’t really justify commercial importance of the material because of difficulties in processing the rigid, sometimes brittle polymer.
Then came along Mr. Waldo Semon of B.F Goodrich Company (USA) who in 1926, invented plasticized PVC that could be made into adhesives and sheets. The result was a more flexible and more easily processed material that soon achieved widespread commercial use.
From almost being recessed and abandoned, PVC became the most commercially used plastic especially during the World war 2 to replace traditional material to insulate wiring on military ships.
Today 100s of products are made from PVC such as pipes, toys, appliances etc.



POLYSYTRENE
Polystyrene was first discovered in 1839 by Eduard Simon from Berlin. It was synthesized from its original resin, storax (a resin from the bark of Oriental and/or American sweetgum trees). He distilled an oily substance, a monomer that he named styrol. He later discovered that the styrol thickened into a jelly like substance which he named Styroloxide because he believed it was due to oxidization that styrol had thickened.
This realization came almost a century later when a German chemist Hermann Staudinger theorized that Styrol polymerized due to heat exposure. This eventually led to the substance receiving its present name, polystyrene.
But it was only until 1930, when I G Farben, a trust company of BASF, commercially manufactured polystyrene.
The company had a history in the production of synthetic coal tar dyes, ammonia, and nitrogenous fertilisers, as well as synthetic rubber and PVC.
Shortly afterwards Dow Chemical introduced polystyrene to the U.S. market in 1937. Styrofoam®, which is a Dow Chemical Co. trademarked form of polystyrene foam insulation and the most recognisable form of foam polystyrene packaging.


PET
Back in DuPont Julian Hill, a close colleague of Wallace Carothers, produced thermoplastic polyesters from which he could pull fibers from the molten polymer, but the work was dropped in favor of nylon.
After the invention of Nylon, John Rex Whinfield and James Tennant Dickson of Calico Printers’ Association of Manchester, England who patented it in 1941, were doing research on polymers that could be used to make fibers.
It wasn’t until 1967 that Nathaniel Wyeth, a Dupont engineer started looking at the potential for carbonated drinks to be stored in plastic bottles.
He initially experimented with a plastic detergent bottle but realised that a stronger material was needed to cope with the pressure of the contents. He eventually opted for PET and received the patent for bottles in 1973.


ABS
Acrylonitrile Butadiene Styrene became available during the World War 2. Scientists were attempting to create a bulletproof plastic sheets using polymer systems where special butadiene acrylonitrile copolymers and styrene acrylonitrile copolymers with high molecular masses were modified by butadiene rubber. Essentially a styrene-acrylonitrile copolymer modified by butadiene rubber, ABS combines the resilience of polybutadiene with the hardness and rigidity of polyacrylonitrile and polystyrene.
ABS polymers have greater toughness over styrene, this made them suitable for many applications, and its limitations led to the introduction of a rubber (butadiene) as a third monomer and hence was born the range of materials popularly referred to as ABS plastics. These became available in the 1950’s and the variability of these copolymers and ease of processing has led to ABS becoming the most popular of the engineering polymers.
PC
Polycarbonate was discovered in 1953 by Bayer’s scientist, Dr. H. Schnell and inadvertently by Daniel W Fox of General Electric in 1960 while working on a wire-coating material.
Bayer’s basic patent claiming polycarbonate products made by either solution or interfacial processes was filed in 1954 and issued in 1962. General Electric’s patent claiming products made by way of transesterification was filed in 1955 and issued in 1964.Bayer began commercial production under the trade name Makrolon in 1958 and GE began production under the name Lexan in 1960.

PP
In 1951, while attempting to convert propylene into gasoline, J. Paul Hogan and Robert L. Banks of Phillips Petroleum Company discovered polypropylene, a high-melting crystalline aliphatic hydrocarbon.


PE
Polyethlene was an accidental discovery. First by a German chemist Hans Von Penchmann in 1898 while investigating diazomethane.
But it was not until 1933 that Eric Fawcett and Reginald Gibson of Imperial Chemical Industries (ICI) who by accident produced a white waxy material when applying high pressure to ethylene benzaldehyde. The first patents for polythene were registered in 1936 by ICI. A year later the first practical use for the material, as a film, was discovered.
In 1953, Karl Ziegler of the Kaiser Wilhelm Institute (renamed the Max Planck Institute) and Erhard Holzkamp invented high-density polyethylene (HDPE). The process included the use of catalysts and low pressure, which is the basis for the formulation of many varieties of polyethylene compounds. Two years later, in 1955, HDPE was produced as pipe. For his successful invention of HDPE, Ziegler was awarded the 1963 Nobel Prize for Chemistry

POM
Polyoxymethylene (POM) was discovered by Hermann Staundinger in the 1920’s, however the material was thermally unstable so not useful commercially.
The inventor of a heat-stable (and therefore useful) POM homopolymer was Stephen Dal Nogare, who discovered that reacting the hemiacetal ends with acetic anhydride converts the readily depolymerizable hemiacetal into a thermally stable, melt-processable plastic.

